Notes
Onsager Conductivity Equation …
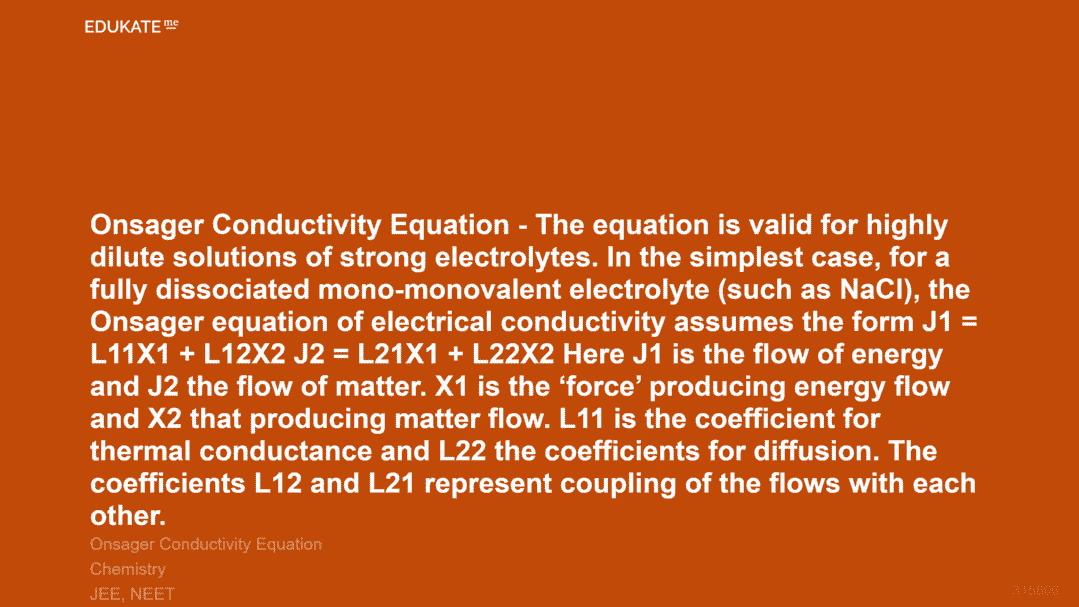
Onsager Conductivity Equation – The equation is valid for highly dilute solutions of strong electrolytes. In the simplest case, for a fully dissociated mono-monovalent electrolyte (such as NaCl), the Onsager equation of electrical conductivity assumes the formJ1 = L11X1 + L12X2
J2 = L21X1 + L22X2
Here J1 is the flow of energy and J2 the flow of matter. X1 is the ‘force’ producing energy flow and X2 that producing matter flow. L11 is the coefficient for thermal conductance and L22 the coefficients for diffusion. The coefficients L12 and L21 represent coupling of the flows with each other.
Subscribe to our youtube channel!
Staying up to date on Question/Notes related to General knowledge, current affairs and are useful in Academic and Government Exams. More than 8000 video on our channel.
Subscribe