Notes
Rules for Writing Resonance Structures in Electronic Displacements in a Covalent Bond…
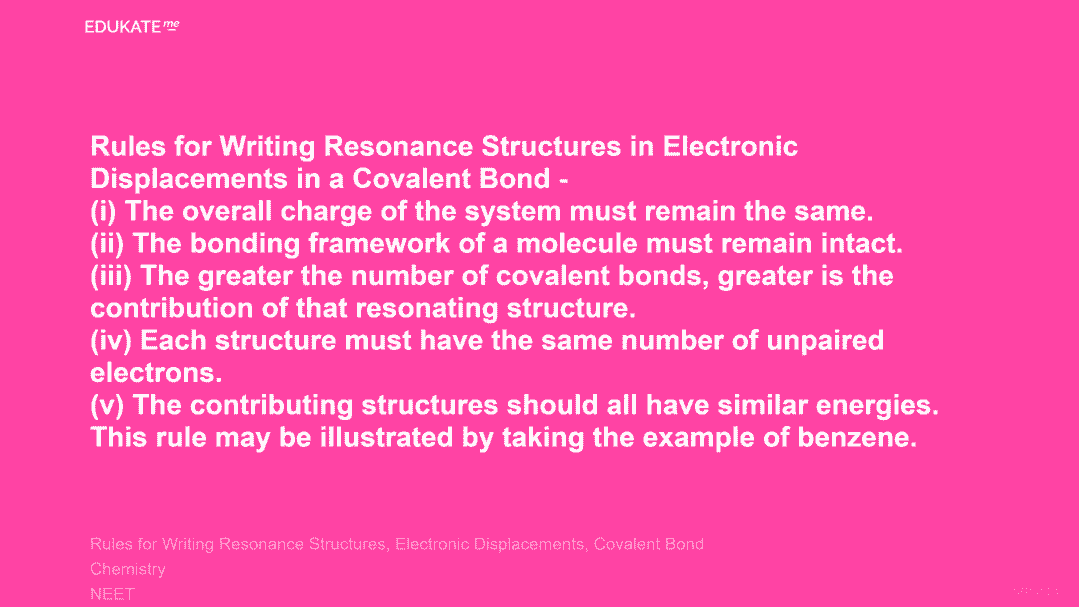
Rules for Writing Resonance Structures in Electronic Displacements in a Covalent Bond –(i) The overall charge of the system must remain the same.
(ii) The bonding framework of a molecule must remain intact.
(iii) The greater the number of covalent bonds, greater is the contribution of that resonating structure.
(iv) Each structure must have the same number of unpaired electrons.
(v) The contributing structures should all have similar energies. This rule may be illustrated by taking the example of benzene.
Subscribe to our youtube channel!
Staying up to date on Question/Notes related to General knowledge, current affairs and are useful in Academic and Government Exams. More than 8000 video on our channel.
Subscribe
Hinglish Excerpt
Rules for Writing Resonance Structures in Electronic Displacements in a Covalent Bond…
Tags: covalent bondElectronic DisplacementsRules for Writing Resonance Structures
Subjects: Chemistry
Exams: NEET